Describe the Mole and Its Use in Chemistry Calculations
It has a mass that is equal to its relative formula mass. At all levels of chemistry ispreparing.

Early Mole Calculation Overview A Level Chemistry Youtube
We used this molar ratio to convert from moles of V.

. In this case logic dictates and the factor-label method supports multiplying the provided amount mol by the molar mass gmol. Chemistry uses a unit called mole. Extending this principle the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in.
Molar mass is a term used to describe the mass of one mole ie. A mole mol is a number of things equal to the number of atoms in exactly 12 g of carbon-12. 1 mol 602214179 10 23 things.
How many moles of copper are present in a sample of copper that has a mass of 213025 g. Number of Atoms or Molecules Number of Moles602210 23 The relationship between the atomic mass unit amu and the gram is given by. Performing calculations using molar relationships is essential to understanding chemistry.
2 76 mol H 2 O 6022 10 23 molecules H 2 O mol H 2 O 1. Experimental measurements have determined that this number is very large. According to the definition of the mole 12 g of 12 C contains 1 mole of 12 C atoms its molar mass is 12 gmol.
Also because we know that there are three atoms in each molecule of H 2 O we can also determine the number of atoms in the sample. The result is in agreement with our. Reactions are balanced based on the number of moles of each element in the reaction solution concentrations are very often described in terms of moles per liter or moles per kg of solvent and we have already seen that the molecules or.
O told us that there are two moles of vanadium V in each mole of V. This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C. 1 mol 602214179 10 23 things.
How many moles of ammonia produced when 6 mole of hydrogen gas react with an excess nitrogen gas. A mole of a molecular compound contains 6 x 10 23 molecules. Feel free to elaborate with equations etc.
92104 mol Ar3995 g mol Ar 0037 g Ar 92 10 4 mol Ar 3995 g mol Ar 0037 g Ar. In this chapter we encounter questions that focus instead on chemical reactions. Experimental measurements have determined that this number is very large.
Sometimes it is more convenient to make calculations with millimoles mmol rather than moles. Chemistry Unit 5 The Mole Answer Key15 Chemistry Unit 5 The Mole Answer Key25 35 Unit 5 Packet. This formula can be written as.
Or you could say 2 moles of ammonia is formed from 1 mole of nitrogen molecules N2 and 3 moles of. Because of its importance the concept of the mole its. The mole chemistry 1.
The relative atomicformulamolecular mass in grams g. Extending this principle the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in. 3 H2 N2 2 NH3 6mol H2 g x 2mol NH3 3mol H2 GIVEN.
The mole is a unit that is used to quantitatively measures the amount of substance. Per the amu definition a single 12 C atom weighs 12 amu its atomic mass is 12 amu. The molar mass of any substance is numerically equivalent to its atomic or formula weight in amu.
A mole is the amount of substance contained in 60221023. MOLE MASS grams OF AN ELEMENT Example 2. The mole is the basic counting unit used in chemistry and is used to keep track of the amount of matter being measured or transferred.
First define it then give examples then why its so important. Millimoles of a species can be determined from its mass in grams or from the mass of a chemically related species. Chemistry uses a unit called mole.
The definition of a mole is an equality that can be used to construct a conversion factor. So a mole of water H 2 O has a mass of 18 g. WHAT IS A MOLE.
According to the definition of the mole 12 g of 12 C contains 1 mole of 12 C atoms its molar mass is 12 gmol. The provided mass of glycine 28 g is a bit more than one-third the molar mass 75 gmol so we would expect the computed result to be a bit greater than one-third of a mole 033 mol. Describe the laboratory technique of heating to constant mass.
INTRODUCTION TO THE MOLE chemistry 2. You use the PERIODIC TABLE to determine the mass of a given element. The coefficients in a balanced chemical equation show the mole ratio of the substances in the reaction.
1 the mass found in one mole of its particles. To moles of vanadium. Set-up all problems Feb 14 2020 Molality m is defined as the number of moles of solute per kilogram of.
You can use the relationship between mole and mass from the periodic table to perform the following calculations. These questions ask us to convert from amount of one substance in a given chemical. Give your answers with four significant digits and correct units.
Moles of ammonia 4mol of NH3. Number of Moles Mass of the SampleMolar Mass The total number of atomsmolecules in a sample can be calculated by multiplying the number of moles with the Avogadro constant. 6 moles of Hydrogen gas.
Give the molar mass for H Sc As I and U. The mass of a mole of substance can be found by expressing the objects mass in atomic mass unit and convert it to grams. 66 1 0 24 molecules H 2 O.
One mole of glycine C 2 H 5 O 2 N contains 2 moles of carbon 5 moles of hydrogen 2 moles of oxygen and 1 mole of nitrogen. A mole of 12 C weighs 12 g its molar mass is 12 gmol. A mole mol is a number of things equal to the number of atoms in exactly 12 g of carbon-12.
The millimole is 11000 of a mole and the mass in grams of a millimole the millimolar mass mM is likewise 11000 of the molar mass. Science Chemistry QA Library Describe a mole used in chemistry. 1 mole of ammonia NH3 Consists of 1 mole of nitrogen atoms combined with 3 moles of hydrogen atoms.
This relationship holds for all elements since their atomic masses are measured relative to that of the amu-reference substance 12 C. The reason the mole is so important is because we use the mole as the unit for most of the relationships in chemistry. 1 mol of any substance contains 6022 x 1023 particles or molecules.
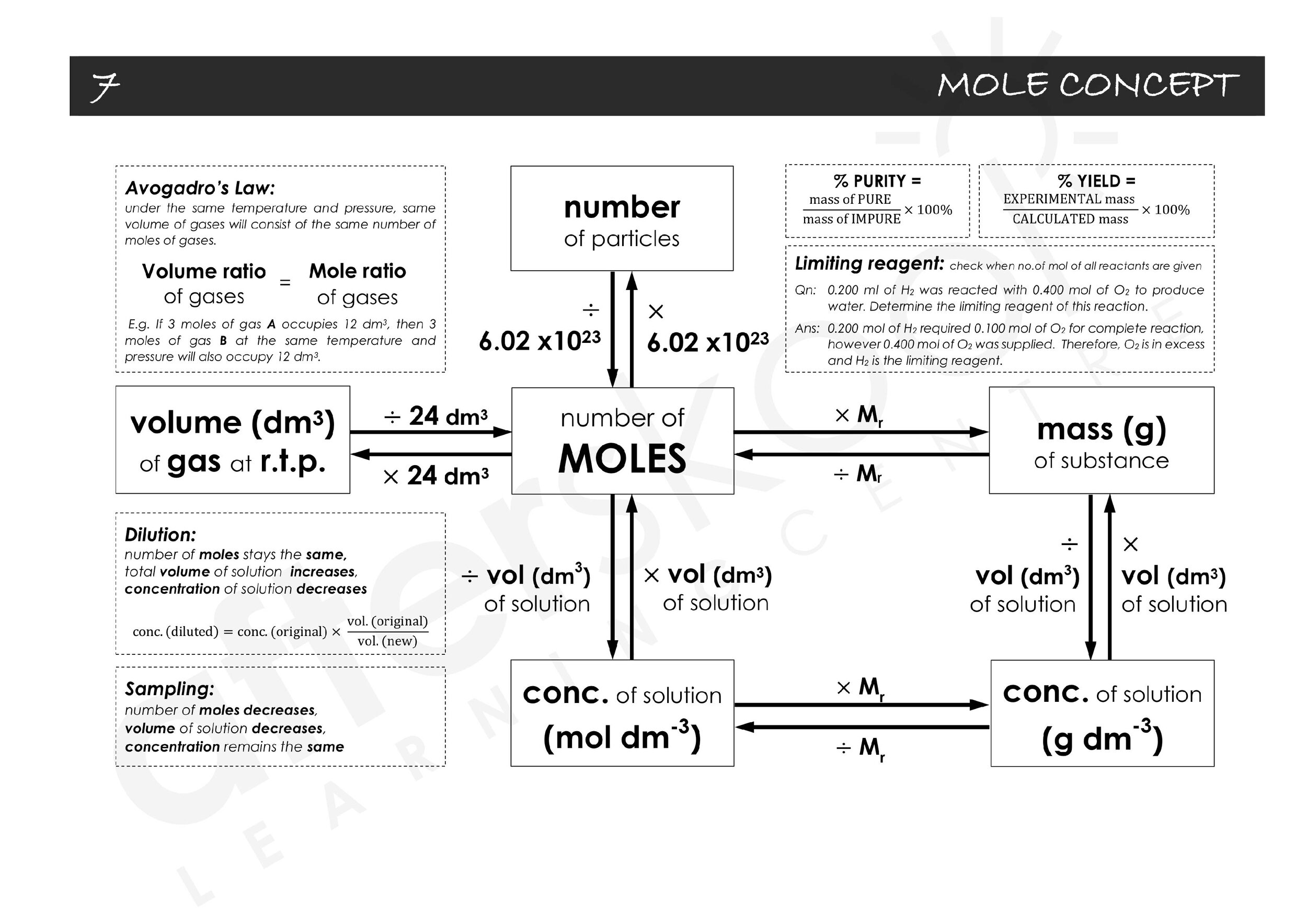
O Level Chemistry 101 Mole Concept Summary Guide Afterskool Learning Centre

Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy

How To Solve Most Mole Calculation Questions Part 1 O Level Chemistry Tuition Leading Chemistry Tuition Centre

Gcse Science Revision Chemistry Calculating Moles Of An Element Youtube

The Mole In Chemical Formulas Youtube

Mole Fraction Formula And Calculation
Calculations Mr Copil S Igcse Chemistry Course

The Mole Chemistry The Mole Very Important Concept Used In Almost All Calculation In Chemistry What Is A Mole A Mole Is The Amount Of Substance That Ppt Download

3 2 Introducing The Mole The Central Unit Of Chemistry Ppt Download
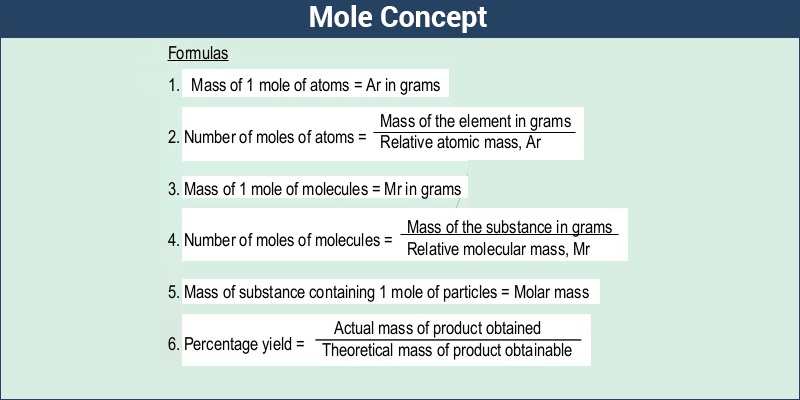
Molar Mass Molecular Weight Definition Formula Examples Of Molar Mass
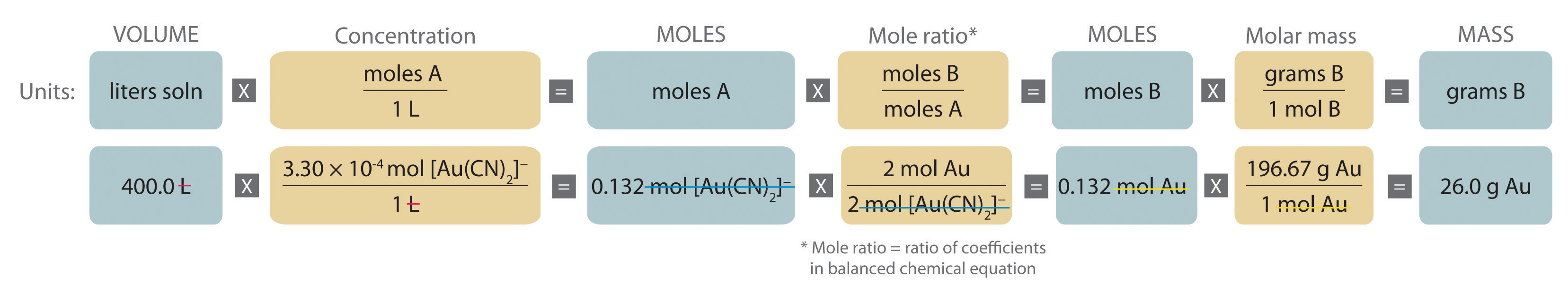
Stoichiometry Of Reactions In Solution

Number Of Moles Formula Definition Concepts And Solved Questions

Moles And Concentration Calculations Gcse Chemistry Lesson Sc9c Chemistry Lessons Gcse Chemistry Chemistry
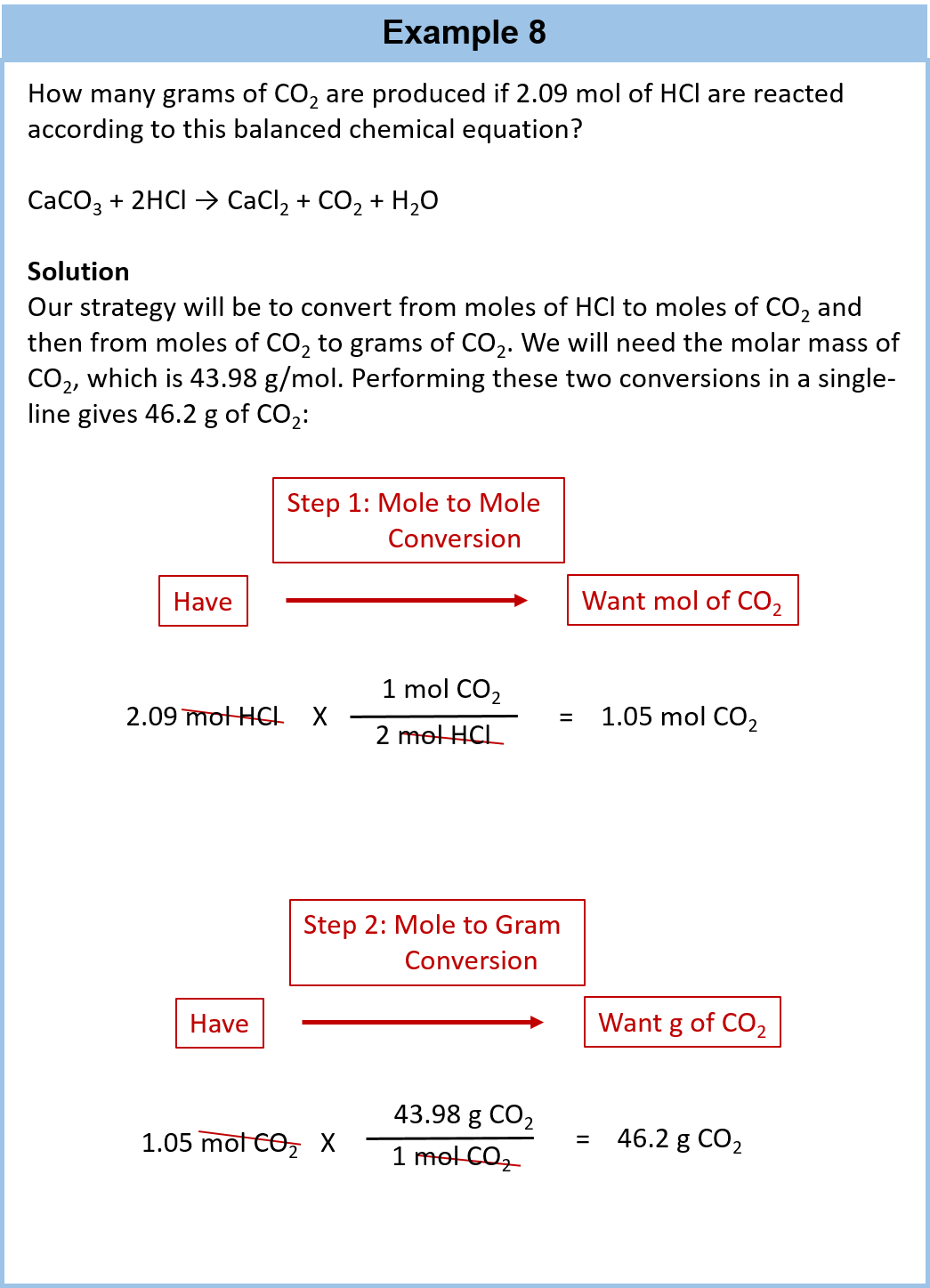
Chapter 6 Quantities In Chemical Reactions Chemistry
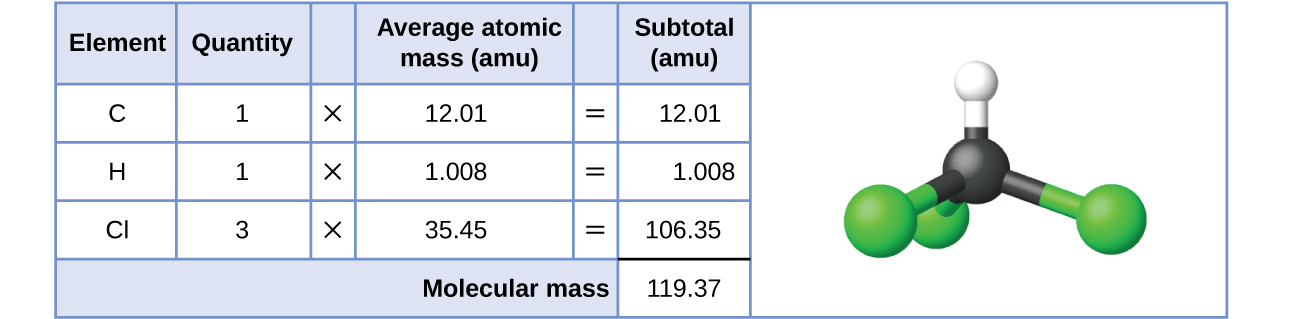
Formula Mass And The Mole Concept Chemistry
Gcse Chemistry Year 10 Amount Of Substance Page

Igcse Chemistry Calculations Involving Moles Youtube

Calculation Of Number Of Moles And Number Of Molecules And Atoms

Mole Calculations Explained Formula Mass And Mole Calculations
Comments
Post a Comment